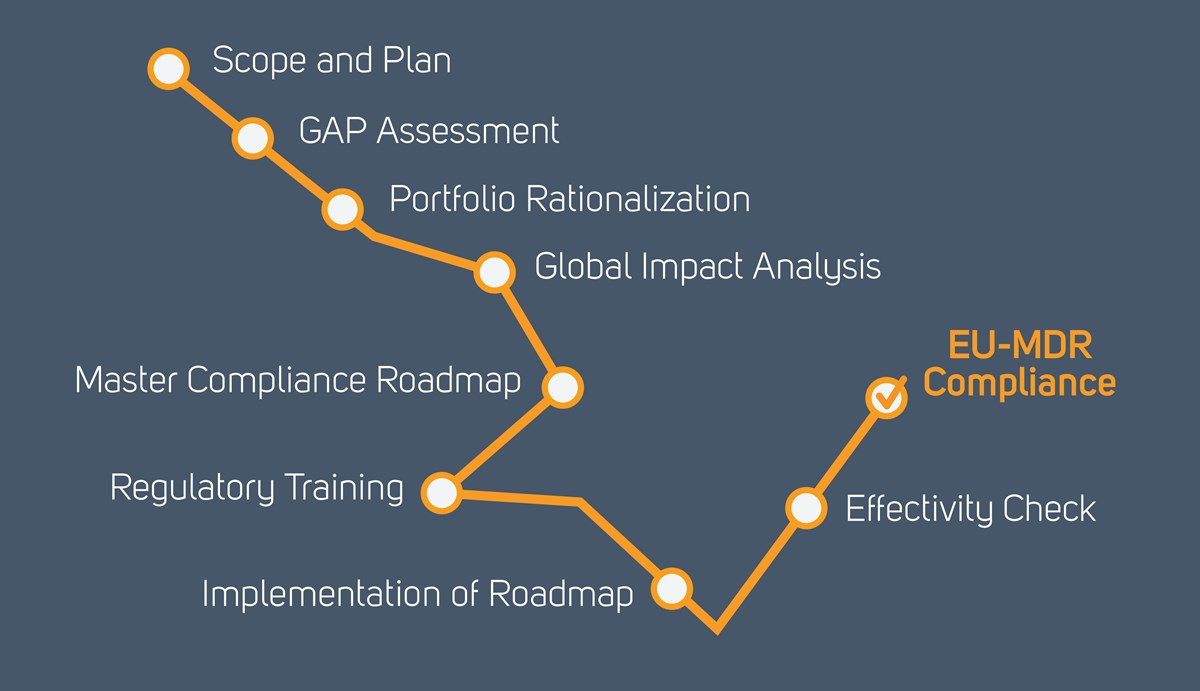
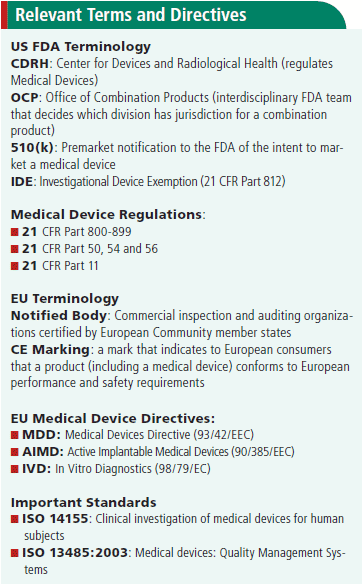
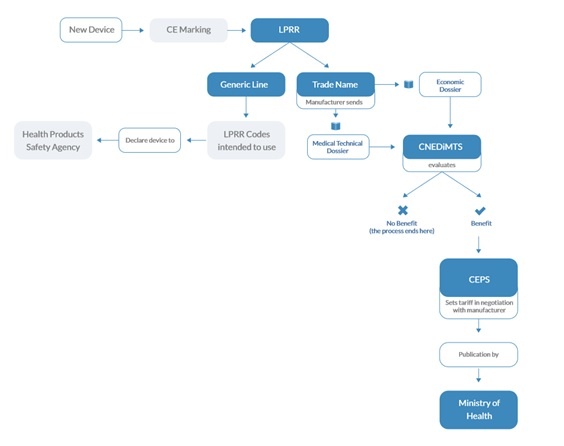
Ce Mark Medical Device Cost
Medical devices of class iia could be such as surgical gloves hearing aids diagnostic ultrasound machines etc.
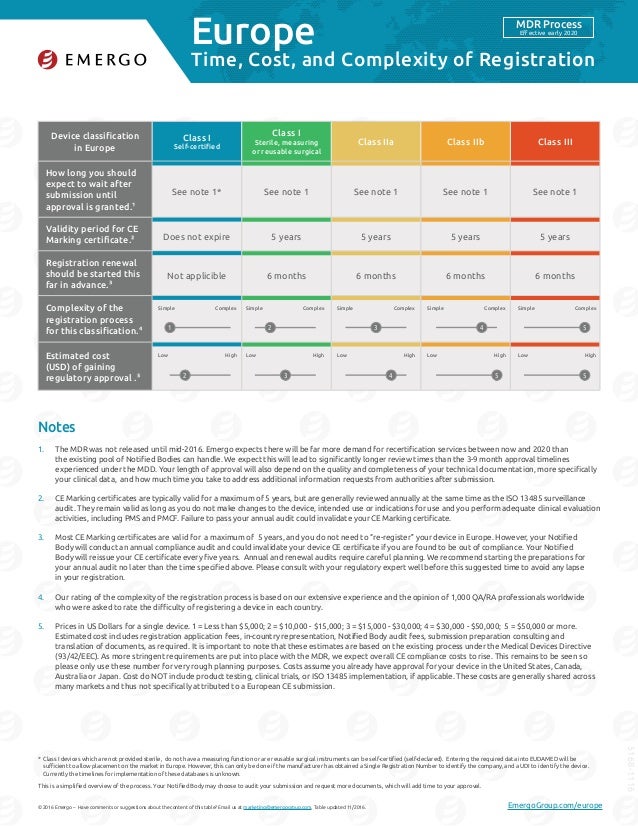
Ce mark medical device cost. Below is i3cglobal s tentative ce certification cost for various classes. Many but not all eu directives require that a product is ce marked. In almost 90 of the cases the ce marking regulations allow products to be self certified for ce marking. Cost of ce marking medical device it is a good idea to know about the cost of ce marking and various factors affecting ce marking cost before you start the ce certification process.
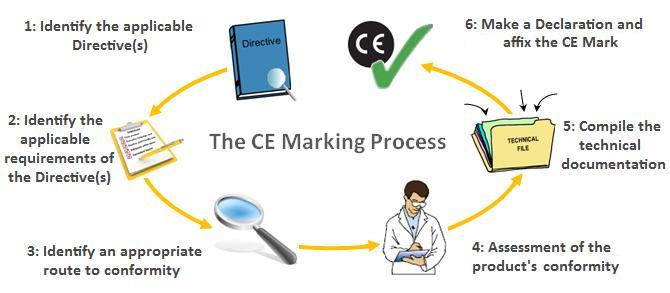
Class iia medical devices. Prepare a declaration of conformity doc which states that your device complies with the appropriate directive. This myth has cost a lot of companies a lot of money. To ce mark a product you must take these steps.
Medical device certification is complex and expensive. The cost and fees involved in the ce marking of medical devices will normally include the follows. The current and new ce marking procedure. You may need to take our final proposal after considering all variants and intended use.
To get ce marking products must be tested and certified by a third party certification body. Ce marking is the medical device manufacturer s claim that a product meets the essential requirements of all relevant european medical device directives. The medical device ce marking process will change when europe s new medical device regulation mdr 2017 745 comes into force in may 2021. They usually constitute low to medium risk.
Cost of ce marking depends on a number of technical files device class intended use constructional material technology. But first let s recap on what ce marking actually is. The directives outline the safety and performance requirements for medical devices in the european union eu. Ce is a framework rather than a standard.
These directives describe the approaches that have to be followed in order to obtain the ce mark. Patients should use them for a short term period any less than 30 days. Currently medical device and in vitro diagnostic developers need to demonstrate that their medical device meets the requirements of respectively directive 93 24 eec and directive 98 79 ec. The cost for ce marking vary from device to device and organization to organization.
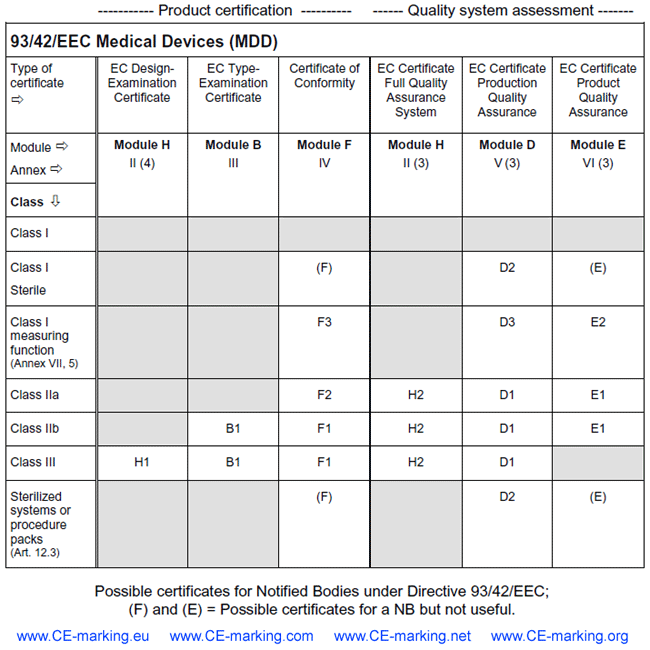
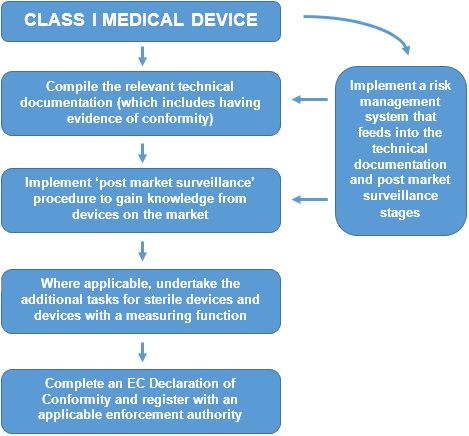
Create the ce label file and make sure that your products get labelled correctly b. Obtain ce marking and iso 13485 certificates from your notified body. Ce marking routes of class i medical devices. Certification fees charged by notified bodies notified bodies always charge fees for issuing ec certifications the fees charged by the notified bodies which are in general profit driven organizations in the private sector varies from notified.
The biggest myth about ce marking is.